Originally posted by Maayanster
View Post
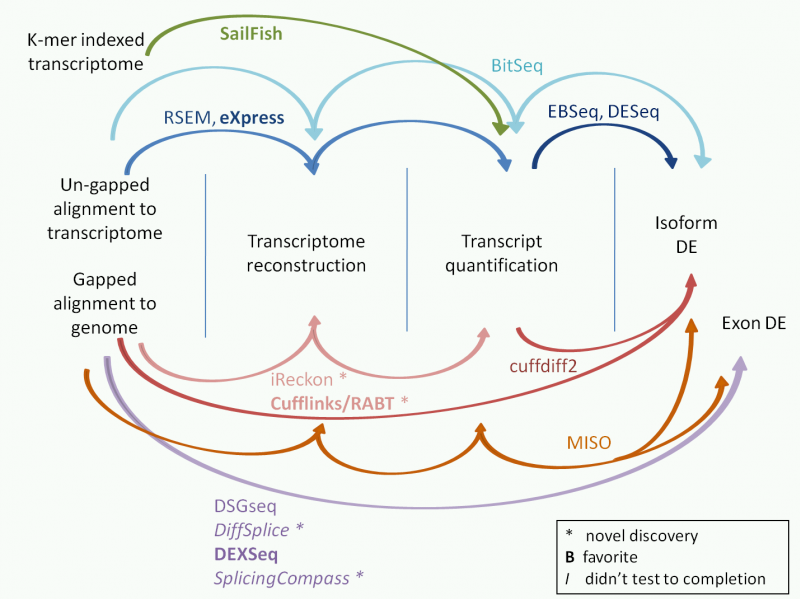
We argue that you do not have to perform transcript/isoform level analysis if the purpose of your analysis is to find genes that change in different conditions (such as knockout and wildtype). As long as you get the read counts for genes using for example a total exon model, you will be able to use the state of the art statistical programs (such as edgeR, limma ...) to perform differential expression analysis. See the Discussion in our latest publication (http://nar.oxfordjournals.org/conten...kt214.abstract).
Cheers,
Wei



Comment